This article was released as Pharm Edaily Premium Content on November 28, 2025, at 9:18 AM.
[Seok Ji-hoen, Edaily Reporter] On Nov. 27, Korea’s biopharmaceutical stocks saw sharp divergences: Vigencell hit the upper price limit after releasing Phase 2 topline data for its NK/T-cell lymphoma therapy, foreign investors piled into Nextbiomedical on signs of U.S. FDA engagement, while Olix fell more than 5% despite reporting positive Phase 1 results for its macular degeneration therapy.
"First-in-world NK/T-cell lymphoma therapy within reach"
According to KG Zeroin MP Doctor (formerly Market Point), Vigencell jumped 29.97% to close at 5,920 won, hitting the upper limit. The surge followed the company’s disclosure of topline results from a Phase 2 trial of its NK/T-cell lymphoma therapy VT-EBV-N.
 |
| Vigencell stock performance (Source: Naver Finance) |
Vigencell said its Phase 2 trial in 48 patients with EBV-positive extranodal NK/T-cell lymphoma (ENKL) showed 2-year disease-free survival (DFS) rates of 95.0% in the treatment arm, compared with 77.58% in the control arm (p=0.0347).
Relapse or death occurred in just 4.76% of treated patients versus 32% in the control group. No deaths occurred in the treatment arm during overall survival (OS) analysis. No serious adverse events (SAEs) were reported.
VT-EBV-N, an autologous NK/T-cell immunotherapy, has been designated an orphan-drug candidate in both Korea and Europe. The latest data allow the company to pursue fast-track review and conditional approval from Korea’s Ministry of Food and Drug Safety (MFDS). A final CSR is expected in the first half of 2026.
Vigencell plans to commercialize the therapy domestically through its exclusive partnership with Boryung, while seeking global licensing deals abroad. If approved, VT-EBV-N would become the world’s first approved cell therapy specifically targeting NK/T-cell lymphoma, a milestone that could elevate the value of Vigencell’s cell-therapy platforms ViTier, ViRanger and ViMedier.
Foreign investors pick Nextbiomedical
Nextbiomedical rose 7.95% to close at 82,800 won, boosted by news that the company recently completed a U.S. FDA Pre-Submission (Pre-sub) meeting for its uterine fibroid embolization material Nexsphere™.
 |
| Nextbiomedical stock performance (Source: Naver Finance) |
Nexsphere is a gelatin-based microsphere that is mixed with contrast agent and delivered through a catheter to temporarily block blood flow to targeted blood vessels during uterine fibroid embolization. The product already holds approvals from Korea’s MFDS and Europe’s CE marking.
The Pre-sub process—conducted prior to formal 510(k) or De Novo applications—focused on device characteristics, clinical-study design and evaluation methods. The company is preparing for entry into what it calls the “three major markets”: Korea, Europe and now the United States.
A company official said, “With FDA discussions now underway for the uterine fibroid indication, we expect Nexsphere’s global value to be recognized more fully. We will continue developing the product with both patient safety and procedural convenience in mind.”
Foreign investors have been active buyers. Morgan Stanley purchased 57,713 shares, topping the day’s buy-side brokerage rankings. It marked the second straight day of foreign net buying, following a 34,833-share net purchase the previous session.
Profit-taking after IR?
Olix fell 5.04% to 124,400 won, despite releasing encouraging U.S. Phase 1 results for its dry and wet age-related macular degeneration (AMD) candidate OLX301A, and presenting an optimistic outlook during an investor relations session earlier in the day.
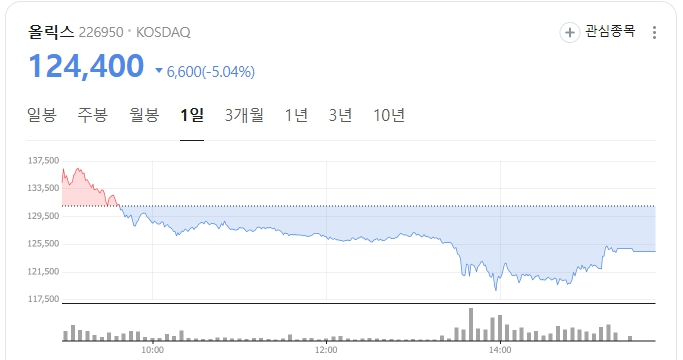 |
| Olix stock performance (Source: Naver Finance) |
Olix announced on Nov. 10 that OLX301A (OLX10212) demonstrated excellent safety and tolerability across all single- and repeat-dose cohorts. No drug-related SAEs or dose-limiting toxicities (DLTs) were observed. No clinically meaningful intraocular-pressure increases or 15-letter BCVA losses occurred. Only one mild ocular inflammatory event was reported.
The trial was conducted at five U.S. sites in a broad AMD population under FDA guidance. The company said it aims to confirm efficacy signals—particularly visual-acuity improvement—in later-stage trials and expand partnerships with global pharmaceutical companies.
Despite the positive data, the stock has trended downward since the announcement: after briefly rebounding, it fell 6.4% on Nov. 11, 1.3% on Nov. 13, and continued declining on Nov. 21, 24 and 25.
Hyundai Motor Securities published a positive note Wednesday, highlighting Olix’s dual delivery platform capable of targeting hepatic and extrahepatic tissues, its partnerships with leading global pharma and beauty companies, and the potential for milestone revenue starting next year.
Still, the stock remained weak. Some market participants suggested that comments made during the IR session may have weighed on sentiment, though this has not been verified.
Olix told Edaily, “There are no special issues tied to the recent share-price movement,” adding, “With our technological competitiveness and strong partnerships, we expect steady growth. We ask the market to focus on upcoming clinical milestones and new deal achievements rather than short-term volatility.”

 1 hour ago
1
1 hour ago
1



![[미리보는 이데일리 신문]AI발 재계 칼바람…부회장부터 줄였다](https://image.edaily.co.kr/images/content/defaultimg.jpg)
![[마켓인] 코스닥·비상장 안 가린다…‘자금력 빈틈’ 파고드는 무자본 M&A](https://image.edaily.co.kr/images/Photo/files/NP/S/2025/12/PS25120401412.800x.0.png)



![[ET특징주]엔씨소프트, 신작 아이온2 흥행에 상승세](https://img.etnews.com/news/article/2025/11/24/news-p.v1.20251124.3f89f49055a64f31beea4a57dacad7c0_P1.gif)

![[마켓인]트러스톤, 태광산업 EB 관련 가처분 취하…“발행 철회 환영”](https://image.edaily.co.kr/images/Photo/files/NP/S/2025/11/PS25112400661.800x.0.png)





 English (US) ·
English (US) ·